
Cytocatch CTC Liquid Biopsy
In collaboration with Delee Corp, I was responsible for assembling a multidisciplinary team of engineers to create a medical device capable of detecting Circulating Tumor Cells or CTCs. These cells are responsible for cancer metastasis in the body as they travel through the bloodstream. It is known that 90% of cancer deaths are due to metastasis in patients; therefore, understanding how and when these cells move through the body can help us not only diagnose the disease but also understand the progression of any treatment in a non-invasive manner.
1. Basic Research
The capture approach employed by the CytoCatch™ was developed by M.Sc. J. Yee de León and myself, and it is based on the differences in size and deformability between blood cells and CTCs. This allows the capture of CTCs whose epithelial phenotype is downregulated, in comparison to technologies that rely on specific antigen-antibody interactions, giving a 96% capture efficiency, far better than the 42% gold standard of the industry.
For further technical information of the scientific principles, please read:
2. Planning & Prototyping
In the initial stages we commenced with a comprehensive literature review and a series of in-depth consultations with medical pathologists, gaining insight into the challenges they faced in their daily practices. With this feedback, we proceeded to a conceptual design phase, using CAD tools and software simulations to visualize and test preliminary ideas. Following the ideation phase, we conducted material selection, emphasizing biocompatibility, ease of use and durability. Rapid prototyping methods, including 3D printing, laser cutting and CNC machining, were employed to bring our initial designs to tangible life. Each prototype underwent a rigorous iterative process of testing and refinement based on both bench testing and user feedback. Simultaneously, we remained vigilant about regulatory guidelines and quality standards to ensure that our developments were compliant from the very inception.
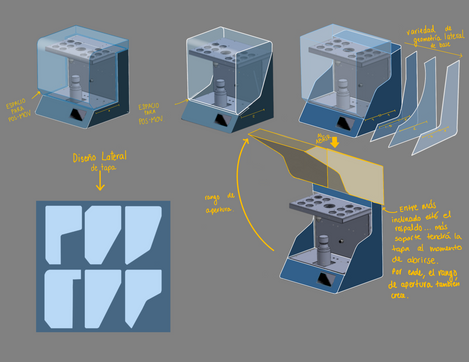





3. Intellectual Property
Throughout our medical device development journey, safeguarding our intellectual property (IP) was of paramount importance. Early in the concept phase, we undertook a thorough patent landscape analysis to ensure our innovations didn't infringe upon existing patents, while also identifying unique aspects of our device for potential patenting. As we refined our designs, we liaised with IP attorneys to file provisional patents, subsequently leading to full patent applications, trademarks, and copyrights as relevant.
4. Design for Manufacturing
Design for Manufacturing (DFM) principles guided our developmental stages, emphasizing the ease of production, material selection, and cost optimization. Collaborating with manufacturers during the design phase, we ensured that our device could be produced efficiently at scale without compromising on quality. For financial feasibility, a comprehensive cost analysis was conducted, factoring in development expenses, manufacturing costs, potential pricing strategies, and market size. Potential revenue streams and return on investment were projected, and different financing models, including venture capital, grants, and internal funding, were evaluated.






5. Quality Assurance
In the realm of medical device development, ensuring quality isn't just a goal—it's an imperative. From the inception of our device, a rigorous Quality Management System (QMS) was established, compliant with industry standards like ISO 13485. Initially, a thorough risk assessment, including Failure Mode and Effects Analysis (FMEA), was conducted to identify potential pitfalls and challenges. As our design matured, prototypes were subjected to meticulous bench testing, simulating real-world conditions and potential stress factors. This was complemented by clinical trials, adhering to stringent protocols to ascertain the device's safety and efficacy in actual medical scenarios. Feedback loops were critical: every test and trial outcome was systematically documented, and any discrepancies led to design modifications followed by re-testing.
6. Market Transition
We established a close collaboration with manufacturing partners early on, enabling us to preemptively address potential scale-up challenges and maintain production quality. By utilizing modular and scalable designs, we ensured that our device could adapt to evolving market demands without necessitating a complete redesign. Continuous training and skill development programs were instituted for our technical team, enabling them to swiftly respond to post-launch feedback and potential product enhancements. Concurrently, we established strong communication channels with regulatory bodies, ensuring our device always met or exceeded compliance standards.




